요약방법최적의 물 추출 발효 공정 변수를 결정하기 위해 단일 요인 및 직교 실험을 진행하였다. 총 다당류 함량에 따라 물질 대 액체 비율, 온도, 물담근 시간을 결정하였다. Saccharomyces cerevisiae의 흡광도를 지표로 하여 발효온도, 교반속도, 접종량, 발효시간을 조사하였다. 물 추출 다당류와 발효 추출 다당류의 분자량 분포를 결정하기 위해 고성능 겔 투과 크로마토그래피를 사용하였다. 다 당류의 항산화 활성은 DPPH와 ABTS 라디칼 소거능, FRAP (철환원 항산화력)을 이용하여 평가하였다.
AbstractPurposeWe wished to determine the optimal method for the extraction of polysaccharides with antioxidant activities from Polygonatum odoratum.
MethodsSingle-factor and orthogonal tests were applied to identify the best parameters for water extraction and fermentation. The solid-liquid ratio, temperature, and duration of water extraction were determined according to the total polysaccharide content. The fermentation temperature, agitation speed, inoculation amount, and fermentation time were investigated according to the optical density of Saccharomyces cerevisiae. The molecular weight distributions of water- and fermentation-extracted polysaccharides were determined by high performance gel permeation chromatography. The antioxidant activities of the polysaccharides were evaluated using 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) ABTS radical scavenging capacities, and ferric reducing antioxidant power (FRAP).
ResultsThe optimum water extraction parameters were 80°C for 2.5 h with a 1:60 solid-liquid ratio. For fermentation, an 8% extraction volume was inoculated with S. cerevisiae. Optimal fermentation occurred at 28°C for 28 h, with an agitation speed of 180 r/min. The molecular weights of water- and fermentation-extracted polysaccharides were 2.067×104 and 9.475×103 Da, respectively. The DPPH, ABTS, and FRAP results demonstrated an obvious increase in antioxidant activity after fermentation.
中文摘要方法 采用单因素试验和正交试验确定最佳水提发酵工艺参数。根据多糖总含量确定料液比、温度和水浸时间。以酿酒酵母的光密度为指标,考察发酵温度、搅拌速度、接种量和发酵时间。采用高效凝胶渗透色谱法测定了水提多糖和发酵提多糖的分子量分布。以DPPH和ABTS自由基清除能力和铁还原抗氧化能力(FRAP)评价多糖的抗氧化活性。
Introduction
Polygonatum odoratum, also called yuzhu, waisheng, weirui, or lingdangcai in Chinese, originated in the Southwest region of China and is well distributed in the wild (Lan et al., 2011). Its rhizomes are widely used as an ingredient/supplement (e.g., in functional foods, flavorings, and teas). It is well known in traditional Chinese medicine (TCM) for removing dryness, promoting the secretion of fluid, and quenching thirst (Deng et al., 2012; Yang et al., 2010).
Previous phytochemical studies have shown that P. odoratum contains a variety of chemical constituents, such as steroidal glycosides, dipeptides, flavonoids, and polysaccharides. P. odoratum polysaccharides (POPs) are of primarily functional composition, with formidable anti-tumor, antioxidant, anti-inflammatory, and immunity improvement effects (Bai et al., 2013; Wang et al., 2009; Guo et al., 2013; Qian et al., 2010; Wang et al., 2013). The microbial fermentation is a process of biotransformation, and has been applied for centuries in TCM. More than a thousand years ago, the Chinese people began to use microbial fermentation to enhance efficacy and potency and reduce toxicity. For example, microorganisms such as Saccharomyces cerevisiae, lactic acid bacteria, and particularly medicinal fungi like Poria cocos, Ophiocordyceps sinensis, and Ganoderma lucidum are used to ferment and transform TCMs, producing new prescriptions with a variety of active ingredients (Li et al., 2004; Wu et al., 2013).
Polysaccharides are the main active ingredients in Polygonatum odoratum and have good antioxidant properties (Cui,2018). The main purpose of this article is to determine the best method for extracting antioxidant POP, in order to improve its antioxidant effect in cosmetics and better develop the efficacy of POPs.
Methods1. Chemicals and reagents
P. odoratum was collected in Hunan province, China. S. cerevisiae (109 colony-forming units [CFU]/mL) was purchased from China General Microbiological Culture Collection Center. Ethylbenzothiazoline-6-sulphonic acid (ABTS; Sigma-Aldrich, USA), 1,1-diphenyl-2-picrylhydrazyl (DPPH; Tokyo Chemical Industry, Japan), potassium peroxydisulfate (Xilong Chemical Co., Ltd., China), TPTz (Adamas Reagent Co., Ltd., China), iron chloride hexahydrate (Shanghai Macklin Biochemical Co., Ltd., China), iron (II) sulfate heptahydrate (Xilong Chemical Co., Ltd., China), hydrochloric acid (Beijing Chemical Works, China), and L-ascorbic acid (J&K Scientific Co., Ltd., China) were all of analytical grade.
2. Extraction methoddsWater extraction: The medicinal material of Polygonatum odoratum is washed, dried in a drying oven (Rotary evaporator; Shanghai Ailang Instrument Co., LTD; China), and crushed by a crusher (Rocking crusher; Guangzhou Xulang machinery Equipment Co., LTD; China) to obtain the medicinal powder. Weigh 10 g powder and add 300mL deionized water at 1:30 (m/V), and extract it twice at 100℃ by hot reflux for 120 min each time. The extract was mixed and filtered through a 0.45 μm filter plate to obtain the polysaccharide extract of Polygonatum odoratum. The extract was concentrated by Rotary evaporator evaporation at 65℃.The flowchart is shown in Figure 1A.
Fermentation process: Polysaccharides were extracted from dried samples using the optimal parameters. The supernatant was collected in a 250 mL vacuum flask with S. cerevisiae medium. The clear liquid was autoclaved at 121℃ for 20 min for sterilization. When the liquid temperature decreased to 30℃, activated S. cerevisiae was inoculated into the liquid and fermentation was initiated in a shaker (Jia et al., 2006). The flowchart is shown in Figure 1B.
Fermentation will be carried out on the polysaccharide extract obtained after optimizing the water extraction conditions. The fermentation method will produce various secondary metabolites, which will significantly change the drug properties and improve its efficacy. By optimizing two extraction methods and comparing the antioxidant effects of polysaccharides obtained, a more suitable extraction method for POPs was determined.
3. Total polysaccharide contentThe total polysaccharide content was determined using the improved phenol-sulfuric acid method (Liu et al., 2009). Measurements were acquired at 490 nm using a microplate reader (SpectraMax 190; Molecular Devices LLC). The total polysaccharide content was calculated as glucose/g using a calibration curve. Samples were analyzed in triplicate.
4. Single-factor tests for optimal water extractionTo obtain the appropriate conditions for maximum polysaccharide extraction, temperatures of 60, 70, 80, 90, and 100℃, liquid-solid ratios of 1:20, 1:30, 1:40, 1:50, and 1:60, and durations of 1.0, 1.5, 2.0, 2.5, and 3.0 h were independently tested. The total polysaccharide content was determined by the phenol-sulfuric acid method.
5. Orthogonal test for water extractionAccording to the results of the single factor experiment, an L9 (34) orthogonal array with three factors at three levels was used to identify optimum conditions for the maximal extracted polysaccharide yield (Choi & Park, 2002; Wang et al., 2015). The used factors and the levels of orthogonal design are shown in Table 1.
6. Fermentation processPolysaccharides were extracted from dried samples using the optimal parameters. The supernatant was collected in a 250 mL vacuum flask with S. cerevisiae medium. The clear liquid was autoclaved at 121℃ for 20 min for sterilization. When the liquid temperature decreased to 30℃, activated S. cerevisiae was inoculated into the liquid and fermentation was initiated in a shaker (Jia et al., 2006; Zhu et al., 2012).
7. Single-factor tests for optimal fermentationTo determine the appropriate conditions for maximum S. cerevisiae growth, inoculation volumes of 2, 4, 6, 8, and 10%; rotary speeds of 100, 120, 140, 160, and 180 r/min; temperatures of 26, 28, 30, 32, 34℃; and the effects of time on the yield of S. cerevisiae were independently tested. The optical density of S. cerevisiae was determined at 600 nm by microplate reader.
8. Orthogonal test for fermentation optimizationAccording to the results of the single factor experiments, an L9 (34) orthogonal array with four factors at three levels was used to determine the optimal conditions for maximal S. cerevisiae yield. The factors used and the levels of orthogonal design are shown in Table 2.
9. Molecular weight distribution assayThe molecular weights of water-and fermentation-extracted polysaccharides were determined using a Waters e2695 Alliance high performance gel permeation chromatography system equipped with a DAWN HELEOS-II Multi-Angle static Light Scattering detector (Wyatt Technologies) and a refractive index detector. All samples (3.0 mg) were dissolved in 0.1 M NaNO3, passed through a 0.45 μm filter, and applied to a Shodex SUGAR KS-805/KS-803 gel-filtration chromatographic column. The column was maintained at 60℃ and eluted with 0.1M NaNO3 at a flow rate of 0.8 mL/min.
10. Determination of DPPH radical scavenging activityDPPH radical scavenging rates in the extracts were measured according to Brand et al. (1995). Samples (1.0, 2.0, 4.0, 6.0, 8.0, and 10.0 mg/mL) were added to 1.0 mL of 0.2 mM ethanolic DPPH and shaken. After 30 min, the decrease in absorbance was measured at 517 nm. Methanol was used as a blank, and ascorbic acid was used as a positive control. Assays were performed in triplicate.
11. Scavenging of ABTS radicalsThe radical scavenging activities of the extracts against ABTS radical cations were measured using the method of Li et al. (2012) with some modifications. ABTS was dissolved in water to 7 mmol/L. ABTS radical cations were produced by reacting an ABTS stock solution with 2.45 mmol/L potassium persulfate and allowing the mixture to stand in the dark at 25℃ for 12-16 h before use. The ABTS radical cation solution was diluted in ethanol to an absorbance of 0.70±0.02 at 734 nm and equilibrated at 30℃. Samples (0.1 mL; final concentrations of 0.1, 0.5, 1.0, 2.0, 4.0, and 6.0 mg/mL) were mixed with 3.9 mL of diluted ABTS radical cation solution, and the absorbance at 734 nm was measured after reaction for 6 min.
12. Total antioxidant capacity assayThe total antioxidant capacity of the extracts was measured by the ferric reducing antioxidant power (FRAP) method (Wang et al., 2021; Nilsson et al., 2005). We added 180 μL FRAP working liquid into each well of a 96-well plate, then added 5.0 μL of FeSO4 standard solution at concentrations of 0.15, 0.3, 0.6, 0.9, 1.2, and 1.5 mM to the standard curve detection wells. Sample wells received 5.0 μL of the extracted samples in triplicate, and a 0.15 mM sample containing 1.5 mM Trolox was used as a positive control. The plate was gently shaken and incubated at 37℃ for 5-7 minutes, and then the decrease in absorbance was measured at 593 nm. Distilled water was used as a blank. The total antioxidant capacity of the sample was calculated according to the standard curve.
Results1. Optimization of extraction conditions for water-soluble polysaccharidesSingle-factor and orthogonal tests were used to determine the optimal extraction conditions for water-soluble polysaccharides. The effects of extraction temperature, ratio of raw material to water, and extraction time on the polysaccharide yield are shown in Figure 2. The optimal temperature was 90℃, and the optimal liquid-solid ratio was 1:50. Considering energy and efficiency, the optimal extraction time was 2.0 h.
An L9 (33) orthogonal array was used to optimize the extraction parameters, including nine experiments corresponding to nine rows and three columns. The results of all tests are shown in Table 3. The F-value was used to qualitatively identify effective factors. The liquid-solid ratio was of highest importance for effective polysaccharide extraction, followed by temperature and extraction time. The optimum extraction parameters were a 1:60 liquid-solid ratio, an 80℃ extraction temperature, and a 2.5 h extraction time. Under optimal conditions, the total polysaccharide contents were 83.49%.
2. Optimization of fermentation conditionsSingle-factor and orthogonal tests were applied to determine the appropriate condition for maximum S. cerevisiae growth. The optimal fermentation time was 24 h. Effects of inoculation volume, rotary speed, and temperature on the fermentation process are shown in Figure 3, and the growth phase of S. cerevisiae is shown in Figure 3D. The optimal inoculation volume was 6%, and the optimal rotary speed was 160 r/min. The most suitable temperature for fermentation was 28℃.
An L9 (34) orthogonal array was used to further optimize the fermentation conditions. The results are shown in Table 4. The best fermentation conditions were an inoculation volume of 8%, a rotary speed of 180 r/min, a 28 h fermentation time, and a temperature of 28℃. The inoculation volume was of highest importance for effective fermentation, followed by rotary speed, fermentation time, and temperature.
3. Molecular weight distributionThe molecular weight distributions of water and fermentation-extracted polysaccharides were 2.067×104 and 9.475×103 Da, respectively, indicating that the molecular weight after fermentation extraction was lower and more homogeneous than that of water-extracted polysaccharides.
4. DPPH radical scavenging activityThe antioxidant capacities of water- and fermentation-extracted polysaccharides were examined by the DPPH method. Polysaccharides extracted by water and fermentation exhibited DPPH scavenging activity with concentrations showing 50% inhibition (IC50 values) of 4.9 and 1.0 mg/mL, respectively. Fermentation produced better DPPH radical scavenging activity than water extraction (Figure 4A).
5. ABTS radical scavenging activityABTS is converted to its radical cation (ABTS•+) by reacting with a strong oxidizing agent (e.g., potassium permanganate, potassium persulfate). This radical cation is blue and absorbs light at 734 nm, and can be converted back to its colorless neutral form by hydrogen-donating antioxidants. The ABTS•+ inhibitive efficiencies of water- and fermentation-extracted polysaccharides are presented in Figure 4B. Water-and fermentation-extracted polysaccharides showed ABTS radical scavenging activity, with IC50 values of 1.4 and 0.3 mg/mL, respectively. Fermentation produced better ABTS radical scavenging activity than water extraction.
6. Total antioxidant capacityThe total antioxidant capacities of water- and fermentation-extracted polysaccharides (0 to 10 mg/mL) were examined by FRAP. The standard curve provided the equation y=0.411x -0.0755 (R2=0.9946, x: concentration of FeSO4 (mM); y: absorbance). The total antioxidant capacity of each sample was expressed as the FeSO4 concentration. Fermentation produced better total antioxidant capacity than water extraction (Figure 4C, 4D).
DiscussionMaceration, heat reflux, ultrasound assistance, and acidic hydrolysis are common methods for extracting polysaccharides from plant tissues, but they require long extraction times, high extraction temperatures, or expensive equipment. In addition, they may pollute the environment (Qian, 2014). Fermentation is one of extraction method highly influenced by various parameters including the nature of the substrate, pH of the medium, nutrient availability, inducer supplementation, fermentation temperature, etc. (Singhania et al., 2010). Compared with traditional solvent extraction method, fermented small molecule has advantage in skin absorption, and the fermentation process is environmental friendly.
P. odoratum is rich in dietary fibers, whose functional properties are closely related to some of its therapeutic effects, such as reducing the risk of coronary heart disease, diabetes, obesity, and some forms of cancer, and lowering cholesterol and fat levels (Liu et al., 2015; Lan et al., 2012). Most dietary fibers are components of plant cell wall polysaccharides, which are resistant to digestion by the alimentary enzymes of humans (Bhat, 2000). S. cerevisiae is commonly used to ferment materials. Fermented foods or traditional medicines are the result of extensive microbial growth, during which microbial metabolism and biosynthesis pathways transform the starting materials to products with distinct organoleptic properties (Wolfe & Dutton, 2015).
In the present study, the antioxidant activities of POPs were obviously increased by fermentation. Molecular weight distribution analysis indicated that polysaccharide hydrolysis during fermentation increased antioxidant activities. Therefore, fermentation is a preferred technique to extract antioxidant POPs. Subsequent research can apply the fermented polysaccharides extracted from Polygonatum odoratum to cosmetics with anti-aging effects, and use human experiments or mouse skin model experiments to test their antioxidant effects, further demonstrating their efficacy in products.
ConclusionIn this study, we developed an effective fermentation-based extraction method for POPs using single-factor and orthogonal tests. The optimal fermentation method used an extraction inoculation volume of 8%, rotary speed of 180 r/min, fermentation time of 28 h, and fermentation temperature of 28℃. During fermentation, the decreased molecular weight of the POPs produced was consistent with increased antioxidant activity. Our results suggest the potential use of fermented POPs as a material in foods, medicines, or cosmetics.
AcknowledgementsThis work was supported by the National Natural Science Foundations of China (31501402). It was also supported by the Key Laboratory of Cosmetic, China National Light Industry, and Beijing Technology and Business University Innovation Fund ID: KLC-2017-YB3.
NOTESAuthor's contribution
LL and MG designed this study. JL conducted research. ZG and HH provided help and suggestions for this study. JL analyzed these data and wrote the manuscript. All authors
contributed to the editorial changes in the manuscript. All authors read and approved the final manuscript.
Figure 2.The contents of total polysaccharide of single-factor test for water extraction.(A) Temperature; (B) Solid-liquid ratio, (C) Extraction time. The solid-liquid ratio and extraction time remain unchanged by changing the extraction temperature: 60, 70, 80, 90, 100°C; The extraction temperature and extraction time were unchanged by changing the solid-liquid ratio: 20, 30, 40, 50, 60; The extraction temperature and solid-liquid ratio were unchanged by changing the extraction time: 1.0, 1.5, 2.0, 2.5, 3.0 h, and the improved phenol-sulfuric acid method was used to test the polysaccharide content in the extract, so as to determine the optimal extraction temperature, extraction time and solid-liquid ratio.Descriptive statistical analysis was performed on the data.
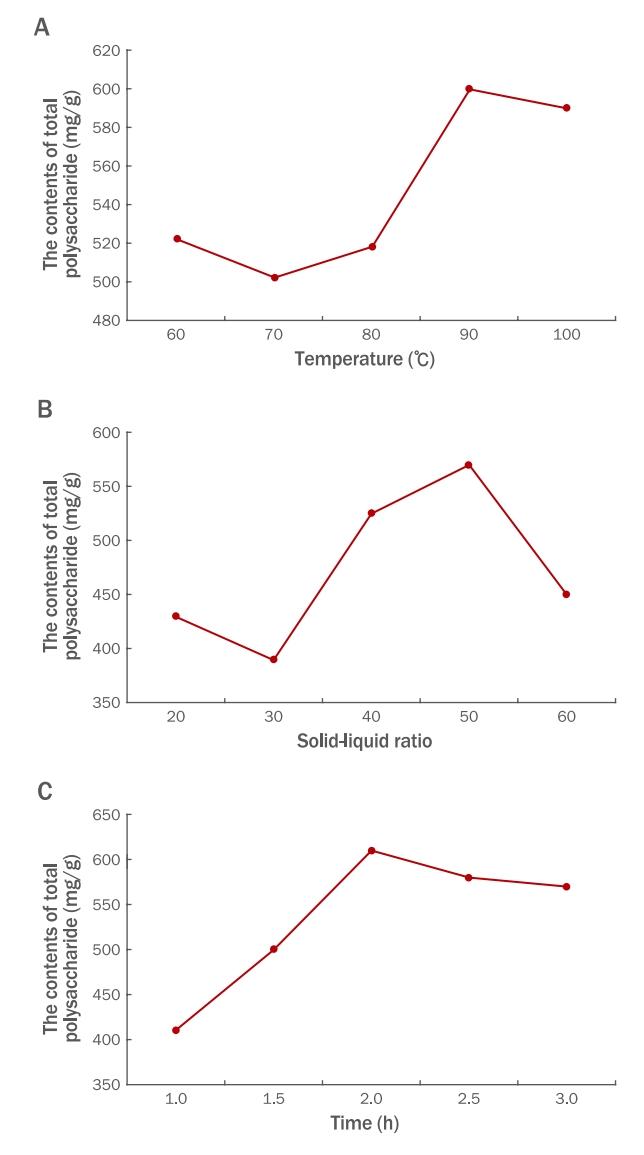
Figure 3.Single-factor test for fermentation parameters on the growth of S.cerevisiae.(A) Inoculation amount; (B) rotary speed; (C) temperature; (D) time. Univariate and orthogonal experiments were used to determine the optimal incubation temperature, time, rotation speed and inoculum amount for culturing Saccharomyces cerevisiae, and descriptive statistical analysis was performed on the data.

Figure 4.Radical scavenging activity and total antioxidant capacity of water- and fermentation-extracted polysaccharides.(A) DPPH free radical scavenging assay was used to study the antioxidant effect of polysaccharides under different conditions concentrations: 1, 2, 4, 6, 8, and 10 mg/mL. Data are presented as mean±SD. (B) ABTS radical scavenging assays were conducted to investigate the antioxidant effect of polysaccharides under different conditions concentrations: 0.1, 0.5, 1, 2, 4 and 6 mg/mL. Data are presented as mean±SD. (C) Standard curve of total antioxidant power. (D) Total antioxidant capacity. Sample A: water polysaccharides; Sample B: fermentation-extracted polysaccharide (n=3).The total antioxidant capacities of water- and fermentation-extracted polysaccharides (0 to 10 mg/mL) were examined by FRAP.
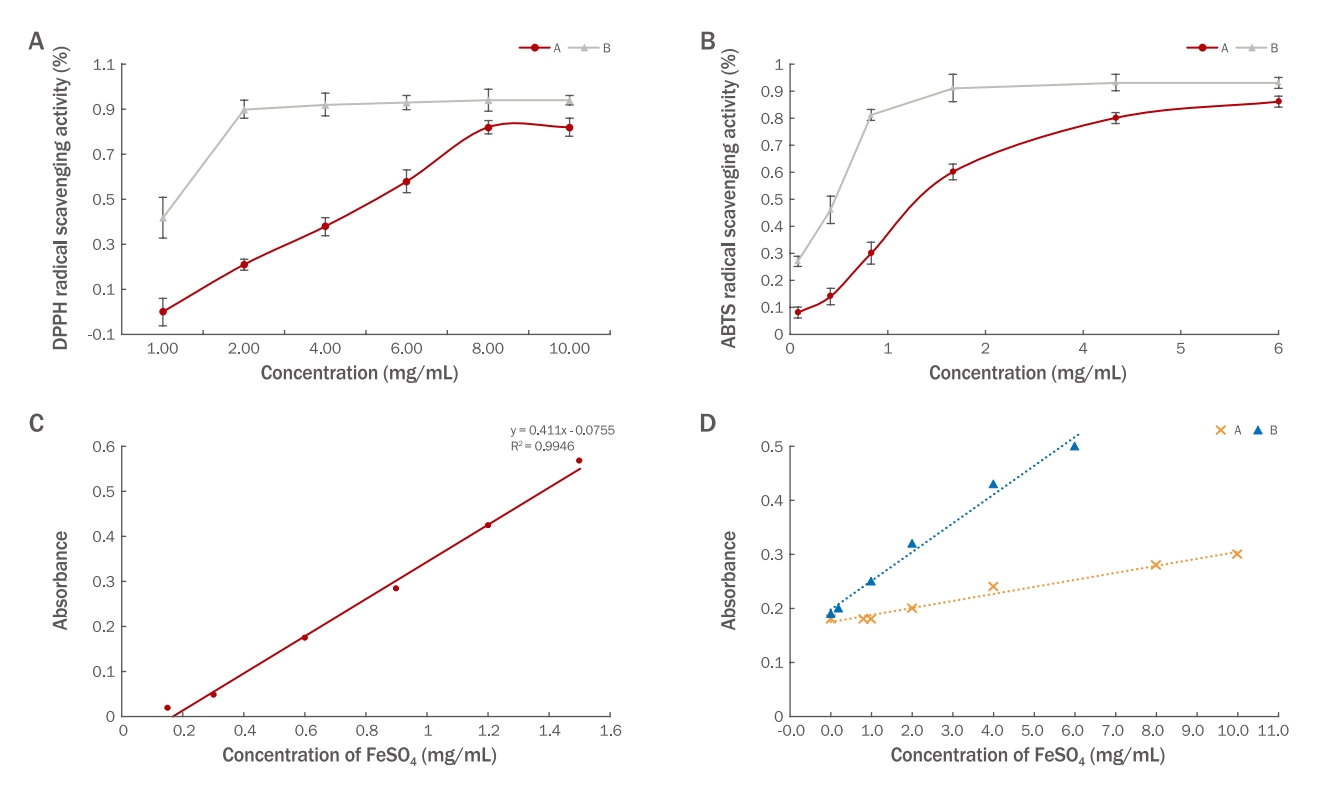
Table 1.Orthogonal test for water extraction
Table 2.Orthogonal test for fermentation parameters
Table 3.Results and analysis of orthogonal test Table 4.Results and analysis of orthogonal test ReferencesBai H, Li W, Zhao HX, Anzai Y, Li HM, Guo HJ, Kato F, Koike K. Isolation and structural elucidation of novel cholestane glycosides and spirostane saponins from Polygonatum odoratum. Steroids 80: 7-14. 2013.
Brand WW, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology 28: 25-30. 1995.
Choi SB, Park S. The effects of water extract of Polygonatum odoratum (Mill) druce on insulin resistance in 90% pancreatectomized rats. Journal of Food Science 67: 2375-2379. 2002.
Cui X, Wang S, Cao H, Guo H, Li Y, Xu F, Zheng M, Xi X, Han C. A review: the bioactivities and pharmacological applications of Polygonatum sibiricum polysaccharides. Molecules 23: 1170. 2018.
Deng Y, He K, Ye X, Chen X, Huang J, Li X, Yuan L, Jin Y, Jin Q, Li P. Saponin rich fractions from Polygonatum odoratum, (MillDruce with more potential hypoglycemic effects. Journal of Ethnopharmacology 141: 228-233. 2012.
Guo H, Zhao H, Kanno Y, Li W, Mu Y, Kuang X, Inouye Y, Koike K, Jiang H, Bai H. A dihydrochalcone and several homoisoflavonoids from Polygonatum odoratum, are activators of adenosine monophosphate activated protein kinase. Bioorganic & Medicinal Chemistry Letters 23: 3137-3139. 2013.
Jia SR, Yin HS, Deng GF, Li Z. Studies on the submerged culture conditions of schizophyllum commune. Journal of Chinese Institute of Food Science and Technology 6: 46-49. 2006.
Lan GS, Chen HX, Chen SH, Tian JG. Chemical composition and physicochemical properties of dietary fiber from Polygonatum odoratum as affected by different processing methods. Food Research International 49: 406-410. 2012.
Lan G, Chen H, Wang Z, Zhang W, Zhang L. Extraction of Polygonatum odoratum, polysaccharides using response surface methodology and preparation of a compound beverage. Carbohydrate Polymers 86: 1175-1180. 2011.
Liu XH, Chen YG, Lin L, Zhuang MX, Fang XJ. Comparison of methods in determination of polysaccharide in Lycium barbarum L. Food Science and Technology 34: 270-272. 2009.
Li X, Lin J, Han W, Mai W, Wang L, Li Q, Lin M, Bai M, Zhang L, Chen D. Antioxidant ability and mechanism of rhizoma Atractylodes macrocephala. Molecules 17: 13457-13472. 2012.
Li Y, Liu ZR, Wu QQ, Jiang YP, Wan DG. Fermented TCM, to open a new field of TCM research & development. Natural Product Research and Development 16: 179-181. 2004.
Liu X, Zhang MS, Guo K, Jia A, Shi Y, Gao G, Sun Z, Liu CH. Cellulase-assisted extraction, characterization, and bioactivity of polysaccharides from Polygonatum odoratum. International Journal of Biological Macromolecules 75: 258-265. 2015.
Nilsson J, Pillai D, Önning G, Persson C, Nilsson A, Akesson B. Comparison of the 2,2′-azinobis-3-ethylbenzotiazo-line-6-sulfonic acid (ABTS) and ferric reducing anti-oxidant power (FRAP) methods to assess the total antioxidant capacity in extracts of fruit and vegetables. Molecular Nutrition & Food Research 49: 239-246. 2005.
Qian ZG. Cellulase-assisted extraction of polysaccharides from Cucurbita moschata and their antibacterial activity. Carbohydrate Polymers 101: 432-434. 2014.
Qian Y, Liang JY, Qu W, Che YY. Two new homoisoflavanones from Polygonatum odoratum (MillDruce. Chinese Chemical Letters 21: 706-708. 2010.
Singhania RR, Sukumaran RK, Patel AK, Larroche C, Pandey A. Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme and Microbial Technology 46: 541-549. 2010.
Wolfe BE, Dutton RJ. Fermented foods as experimentally tractable microbial ecosystems. Cell 161: 49-55. 2015.
Wang D, Li D, Zhu W, Zhang J, Peng P. Steroidal saponins from the rhizomes of Polygonatum odoratum. Natural Product Research 23: 940-947. 2009.
Wang D, Zeng L, Li D, Pu W. Antioxidant activities of different extracts and homoisoflavanones isolated from the Polygonatum odoratum. Natural Product Research 27: 1111-1114. 2013.
Wang R, Luo Q, Feng Y. Determination of antioxidant capacity of kaempferol by DPPH, ABTS and FRAP. Guangzhou Chemical Industry 49: 58-59. 2021.
Wang XL, Wang F, Jing Y, Wang Y, Lin P, Yang L. Application of orthogonal design to optimize extraction of polysaccharide from Cynomorium songaricum Rupr (Cynomoriaceae). Tropical Journal of Pharmaceutical Research 14: 1175-1181. 2015.
Wu TX, Wang N, Zhang Y, Xu X. Advances in the study on microbial fermentation and transformation of traditional Chinese medicine. African Journal of Microbiology Research 7: 1644-1650. 2013.
Yang Y, Xu HL, Zhang ZT, Liu JJ, Li WW, Ming H, Bao JK. Characterization, molecular cloning, and insilico, analysis of a novel mannose-binding lectin from Polygonatum odoratum, (Mill.) with anti-HSV-II and apoptosis-inducing activities. Phytomedicine: International Journal of Phytotherapy & Phytopharmacology 18: 748-755. 2010.
Zhu SJ, Xiang YY, Luo X, Chai XY, Zhang WZ. Technology optimization in extracting Umbilicaria esculenta polysaccharide by yeast fermentation. Journal of Beijing University of Agriculture 27: 65-68. 2012.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||